Preserving Life by Emulating Nature
X-Therma pioneers a breakthrough biopreservation platform, transforming “living” medicines into on demand treatments. We empower patients with access to safe, reliable organs, engineered tissues, and advanced cell & gene therapies, dismantling the obstacles of time and distance.
Products
For Cell and Tissue Cryopreservation
XT-Thrive®

For Kidney Preservation
XT-ViVo® and TimeSeal®

XT-ViVo® and TimeSeal® received FDA Breakthrough Device Designation in 2022. This turnkey solution stores kidneys for up to 5 days at high subzero temperatures for transplantation.
The Problem
Not Enough Time
90% Unmet Needs
80% Transplantable Organs Left Unused
Just Hours to Keep Organs Alive
1 Per Hour Die on Waitlist
Did you know that 80% of transplantable organs are NOT being transplanted, especially hearts and lungs?
In organ transplantation, we are racing against TIME on Ice every day, battling an extremely slim window where every minute matters. We currently perfuse and store an organ in preservation solution that is kept between 2-8°C in an igloo cooler on wet-ice. This only allows organ survival for less than a day. For the heart, that is an ephemeral 4 hrs. This extremely tight race severely hinders organ accessibility, breeds extreme risk, creates a logistics nightmare for the transplant team and significantly impacts patient survival. To win such a race, we MUST remove TIME from the equation.
The Problem
For Cell Manufacturing, DMSO and Serum No Longer Meet Integrated Demands
Poor Performance
• Poor post-thaw cell survival as low as 15-20%
• Cell functionality impaired
Toxic*
• Profound in vivo toxicity
• Severe injection site pain/neurotoxicity
• Adverse Genotoxicity
Hindered Scalability
• Processing nightmare
• Limited scale-up production
• Batch variation
The Problem
Toxicity of In Vitro Materials
DMSO Adverse Toxicity and Genotoxicity
Poor Functional Recovery <10% (human eggs)

"Look deep into nature, and then you will understand everything better."
- Albert Einstein
As a fundamental principle of nature, reducing temperature extends time, but we had to find a way around the detrimental impact of ice crystals. Inspired by antifreeze proteins (AFPs) produced by arctic species who thrive in sub-zero environments, we developed a chemically defined antifreeze molecule that is hyper effective, non-toxic, biocompatible, and scalable.

X-Therma Showcases Groundbreaking Organ Preservation Technology at Johns Hopkins’ “Hopkins on the Hill” Event
X-Therma proudly announces its participation in the prestigious Hopkins on the Hill event, held on June 11, 2025, in Washington, D.C. The biennial event showcased the most impactful federally funded research and innovations across various fields.

X-Therma Welcomes Biotech Veteran Neil Warma to its Board of Directors
X-Therma announced the appointment of Neil Warma, a seasoned healthcare entrepreneur with over 25 years of experience in managing and advising numerous biotechnology and pharmaceutical companies globally, to its Board of Directors.
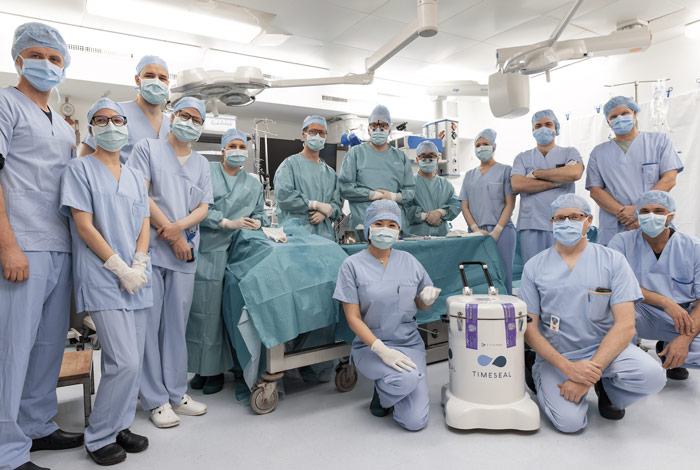
X-Therma Achieves World’s First Subzero Organ Transports: Multiple 48-Hour Transatlantic Journeys Support First Steps Toward Tackling Organ Waitlist
Surgeons successfully transported pig kidneys between Baltimore and Innsbruck five times using X-Therma’s FDA Breakthrough-designated subzero preservation technology