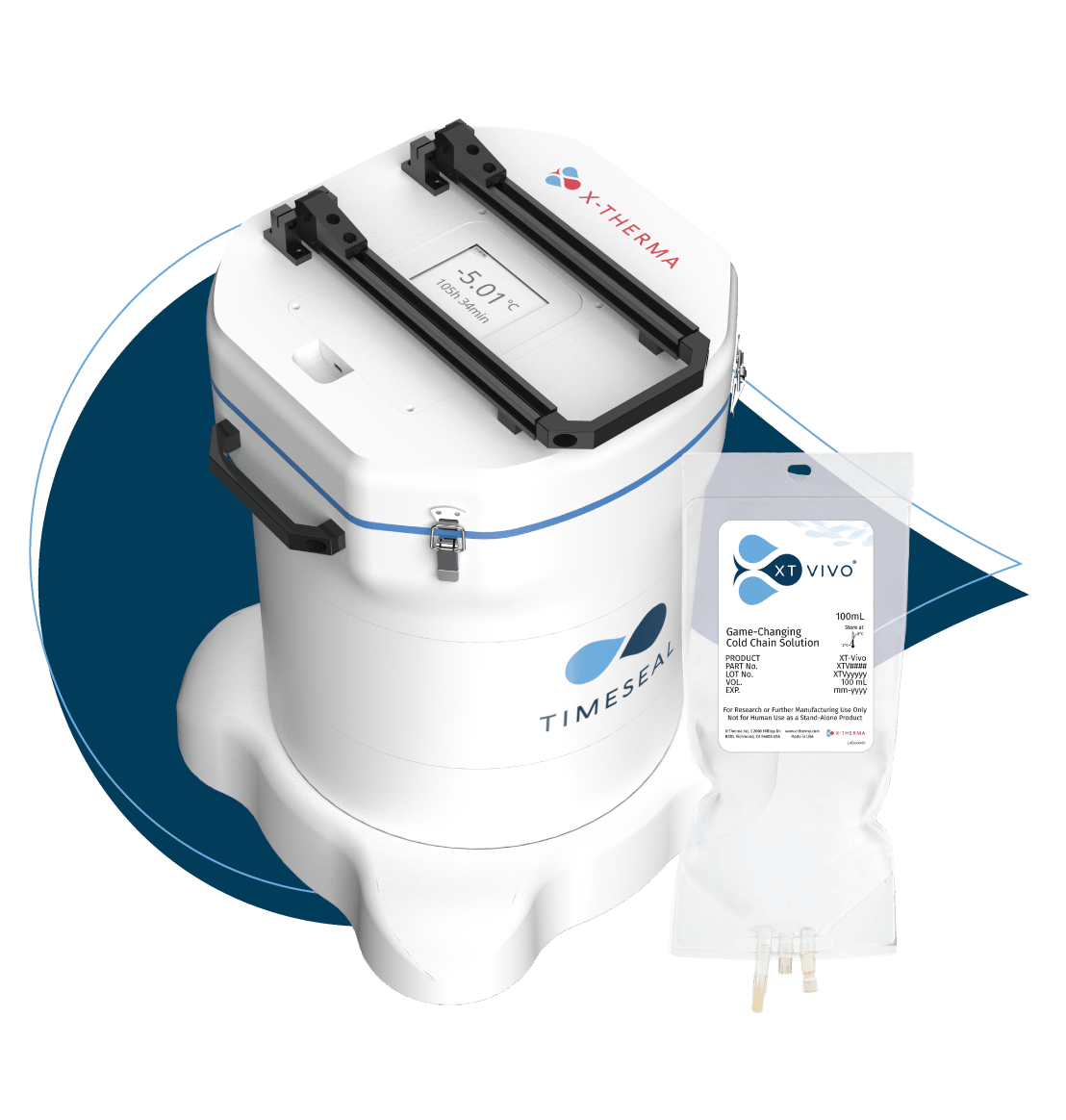
XT-VIVO® & TIMESEAL®
Multi-Day Preservation of Organs, Tissues and Cells
The global shortage of transplantable organs is considered one of the greatest modern medical crises. Only 10% of the worldwide transplantation needs are being met. Every hour in the US, at least one patient dies while waiting for a life-saving organ. Donor availability is one part of the equation… the other is time.
XT-ViVo® & TimeSeal® will enable multi-day preservation and remove time from the equation. Check out our white paper on organ ischemia to learn more!

Organ Transplant
Not Enough Time
Do you know 80% of transplantable organs end up NOT being transplanted today, especially for hearts and lungs?
1/hour
Die on waitlist
80%
transplantable organs left unused
Just hours
to keep organs alive
In organ transplantation, we are racing against TIME on Ice every day, battling an extremely slim window where every minute matters. We currently perfuse and store an organ in preservation solution that is kept between 2-8°C in an igloo cooler on wet-ice. This only allows organ survival for less than a day. For the heart, that is an ephemeral 4 hrs. This extremely tight race severely hinders organ accessibility, breeds extreme risk, creates a logistics nightmare for the transplant team and significantly impacts patient survival.
To win such a race, we MUST remove TIME from the equation.
With X-Therma’s biopreservation breakthroughs, we make a difference.
In Vitro Fertilization
Can we say “No” to toxins for healthy babies?
DMSO
Adverse Toxicity & Genotoxicity
Poor Functional Recovery <10% (human eggs)
In Vitro Fertilization uses a high concentration of toxic vitrification media to freeze eggs or embryos that can kill a cat just in one teaspoon taken orally. This causes severe cell shrinkage and damage to the cell membrane, ultimately leading to a negative impact on the viability of the eggs, giving less than 10% with functional recovery.
The extreme changes in microRNAs and alterations in the epigenetic landscape indicate that DMSO is not inert. Its use should be reconsidered, especially for preservation of embryos and oocytes, since it may impact embryonic development.
Nature, Scientific Reports volume 9, Article number: 4641 (2019)
Organ Transplant
Not Enough Time
Do you know 80% of transplantable organs end up NOT being transplanted today, especially for hearts and lungs?
90%
Die on waitlist
80%
transplantable organs left unused
Just hours
to keep organs alive
In organ transplantation, we are racing against TIME on Ice every day, battling an extremely slim window where every minute matters. We currently perfuse and store an organ in preservation solution that is kept between 2-8°C in an igloo cooler on wet-ice. This only allows organ survival for less than a day. For the heart, that is an ephemeral 4 hrs. This extremely tight race severely hinders organ accessibility, breeds extreme risk, creates a logistics nightmare for the transplant team and significantly impacts patient survival.
To win such a race, we MUST remove TIME from the equation.
In Vitro Fertilization
Can we say “No” to toxins for healthy babies?
DMSO
Adverse Toxicity & Genotoxicity
Poor Functional Recovery <10% (human eggs)
In Vitro Fertilization uses a high concentration of toxic vitrification media to freeze eggs or embryos that can kill a cat just in one teaspoon taken orally. This causes severe cell shrinkage and damage to the cell membrane, ultimately leading to a negative impact on the viability of the eggs, giving less than 10% with functional recovery.
The extreme changes in microRNAs and alterations in the epigenetic landscape indicate that DMSO is not inert. Its use should be reconsidered, especially for preservation of embryos and oocytes, since it may impact embryonic development.
Nature, Scientific Reports volume 9, Article number: 4641 (2019)
With X-Therma’s biopreservation breakthroughs, we make a difference.
You Have Time
Our solutions provide significantly longer preservation of organs, cells, and tissues.
FDA Breakthrough Designation
The FDA has granted XT-ViVo® & TimeSeal® Breakthrough Device Designation for an intended kidney preservation and storage time of up to 120 hours. The products also have the potential to be used in other organ and tissue applications.
Extended Kidney Storage Time Up to 120 Hours


YOU NOW HAVE THE TIME WITH
XT-ViVo® Preservation
XT-ViVo® is a non-toxic, serum- and protein-free, completely defined cold storage solution for kidneys. It consists of fully synthetic biomimetics of antifreeze proteins found in nature and bypasses the drawbacks of toxic molecules. This solution prevents damaging ice crystal formation during storage at subzero temperature while supporting reduced metabolism and ischemic damage.

NON-TOXIC
ICE-FREE
MAKE TRANSPORT EASY WITH
TimeSeal® Smart Sensored Organ Transporter
Effective. Controlled. Simple.
Simple transplant workflow



“No additional steps or equipment were needed. I used exactly the same methods as in clinical kidney transplantation.”
— Gerald Brandacher, MD, FAST; VP of Research and Scientific Director, Johns Hopkins Reconstructive Transplantation Program
Benefits of XT-ViVo® & TimeSeal®
Extension of time
XT-ViVo® will enable subzero preservation for 3-7 days compared to the 4-24 hours we have today.
TimeSeal® will provide a stable subzero environment.
Increased Transplant Availability
XT-ViVo® & TimeSeal® will eliminate geographic restriction, enabling organs, tissues, and cells to be shared around the world.
Better transplant quality
Subzero storage is the most effective way to slow down metabolism and extend the life of a kidney. XT-ViVo® eliminates ice phase transitions and reduces the risk of shock-induced ice damage during organ transport.
Smart & Easy Transportation
The TimeSeal® transport system contains an integrated intelligent sensor system that transmits real-time logistics data to the receiving team during transport. Simplicity and effectiveness in one platform. No supporting accessories such as blood, oxygen, or external power supply are required.
Medical cost reduction
Improved organ matching can shorten postoperative treatment time and reduce medication use.
Organ transplants can be scheduled in advance, eliminating middle-of-the-night and weekend procedures.
new horizons
The ultimate XT-ViVo® and TimeSeal® system removes time from the equation for all regenerative medicines. We can not only transform how transplants are done today, but also offer essential infrastructure that makes centralized recovery, repair and rejuvenation of organs, tissues and cells possible, enabling cutting-edge Xeno-transplants and 3D printed organs to become clinical standard practice. Together, we can accelerate Organs-on-Demand by 10 years and save millions of lives.