Removing Time from the Equation
X-Therma enables the robust preservation of cells, tissues, organs, and other biologics by leveraging our proprietary peptoid. The peptoid inhibits lethal ice crystals, allowing long-term sub-zero storage of biological materials while maintaining unparalleled functionality and viability.

How it Began
When she was 8 years old, Xiaoxi’s beloved grandfather passed away. If he had received a donor liver, he would have had more time to spend with loved ones.
Xiaoxi has since dedicated her life to finding a solution that increases organ availability. In 2014 she obtained her doctorate in medicinal chemistry from SUNY-Buffalo. Soon after, she established X-Therma to bring her vision of eradicating organ waitlists to life.


X-Therma History

2015
Xiaoxi Wei, CEO began her research work for X-Therma at Lawrence Berkeley National Laboratory. She received her first grant awarded to X-Therma. Provisional patent filed.

2016
Only one year later X-Therma had two additional grants in implementation and a team of four. First successful proof of concept for novel preservation of cell lines.

2017
X-Therma now has five grants running simultaneously and filed additional provisional patents.
2018
X-Therma partnered with Johns Hopkins University and Dr. Gerald Brandacher on several grants. X-Therma awarded first patent claim set!

2019
Successful in vivo mouse heart transplant with Johns Hopkins University.
2020
X-Therma survived the Covid-19 pandemic and was able to continue with successful ex vivo organ transplantations.

2021
X-Therma closed an oversubscribed $13M Series A financing led by LOREA AG. The European office was opened.
2022
The FDA designated X-Therma's proprietary organ preservation solution, XT-ViVo®, and TimeSeal® Organ Transport Device, Breakthrough Device status.


News

X-Therma Showcases Groundbreaking Organ Preservation Technology at Johns Hopkins’ “Hopkins on the Hill” Event
X-Therma proudly announces its participation in the prestigious Hopkins on the Hill event, held on June 11, 2025, in Washington, D.C. The biennial event showcased the most impactful federally funded research and innovations across various fields.

X-Therma Welcomes Biotech Veteran Neil Warma to its Board of Directors
X-Therma announced the appointment of Neil Warma, a seasoned healthcare entrepreneur with over 25 years of experience in managing and advising numerous biotechnology and pharmaceutical companies globally, to its Board of Directors.
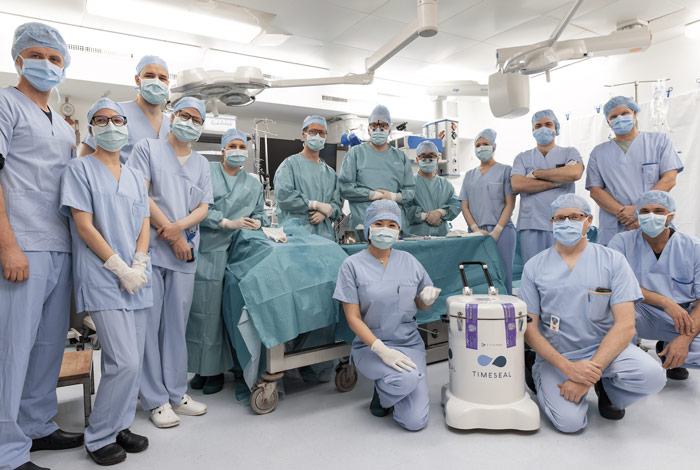
X-Therma Achieves World’s First Subzero Organ Transports: Multiple 48-Hour Transatlantic Journeys Support First Steps Toward Tackling Organ Waitlist
Surgeons successfully transported pig kidneys between Baltimore and Innsbruck five times using X-Therma’s FDA Breakthrough-designated subzero preservation technology