Preserving Life by Emulating Nature
A robust preservation platform for cells, tissues, organs, and regenerative medicines.
We advance regenerative medicine and organ transplantation with a ground-breaking biopreservation platform. We enable patient access to safe and reliable organs, engineered tissues, and cell therapies, breaking down the barriers of time and distance.
You have Time.
Organ Transplant
Not Enough Time
Do you know 80% of transplantable organs end up NOT being transplanted today, especially for hearts and lungs?
90%
1/hour
Die on waitlist
80%
transplantable organs left unused
Just hours
to keep organs alive
In organ transplantation, we are racing against TIME on Ice every day, battling an extremely slim window where every minute matters. We currently perfuse and store an organ in preservation solution that is kept between 2-8°C in an igloo cooler on wet-ice. This only allows organ survival for less than a day. For the heart, that is an ephemeral 4 hrs. This extremely tight race severely hinders organ accessibility, breeds extreme risk, creates a logistics nightmare for the transplant team and significantly impacts patient survival.
To win such a race, we MUST remove TIME from the equation.
With X-Therma’s biopreservation breakthroughs, we make a difference.
cGMP CELL & GENE THERAPY
DMSO and Serum No Longer Meet Integrated Demands
Poor Performance
- Poor post-thaw cell survival as low as 15-20%
- Cell functionality impaired
Toxic
- Profound in vivo toxicity
- Severe injection site pain/neurotoxicity
- Adverse Genotoxicity
Nature, Scientific Reports volume 9, Article number: 4641 (2019)
Hindered Scalability
- Processing nightmare
- Limited scale-up production
- Batch variation
Biopreservation is the key for commercial cell manufacturing & processing, which happens 3-5 times throughout the process. However, preservation has seen little improvement. Existing standards use 5-10% dimethyl sulfoxide (DMSO) with serum. A new solution is needed to enable “off-the-shelf” living medicines.
In Vitro Fertilization
Can we say “No” to toxins for healthy babies?
DMSO
Adverse Toxicity & Genotoxicity
Poor Functional Recovery <10% (human eggs)
In Vitro Fertilization uses a high concentration of toxic vitrification media to freeze eggs or embryos that can kill a cat just in one teaspoon taken orally. This causes severe cell shrinkage and damage to the cell membrane, ultimately leading to a negative impact on the viability of the eggs, giving less than 10% with functional recovery.
The extreme changes in microRNAs and alterations in the epigenetic landscape indicate that DMSO is not inert. Its use should be reconsidered, especially for preservation of embryos and oocytes, since it may impact embryonic development.
Nature, Scientific Reports volume 9, Article number: 4641 (2019)
Organ Transplant
Not Enough Time
Do you know 80% of transplantable organs end up NOT being transplanted today, especially for hearts and lungs?
90%
Die on waitlist
80%
transplantable organs left unused
Just hours
to keep organs alive
In organ transplantation, we are racing against TIME on Ice every day, battling an extremely slim window where every minute matters. We currently perfuse and store an organ in preservation solution that is kept between 2-8°C in an igloo cooler on wet-ice. This only allows organ survival for less than a day. For the heart, that is an ephemeral 4 hrs. This extremely tight race severely hinders organ accessibility, breeds extreme risk, creates a logistics nightmare for the transplant team and significantly impacts patient survival.
To win such a race, we MUST remove TIME from the equation.
cGMP CELL & GENE THERAPY
DMSO and Serum No Longer Meet Integrated Demands
Poor Performance
- Poor post-thaw cell survival as low as 15-20%
- Cell functionality impaired
TOXIC
- Profound in vivo toxicity
- Severe injection site pain/neurotoxicity
- Adverse Genotoxicity
Nature, Scientific Reports volume 9, Article number: 4641 (2019)
HINDERED SCALABILITY
- Processing nightmare
- Limited scale-up production
- Batch variation
Biopreservation is the key for commercial cell manufacturing & processing, which happens 3-5 times throughout the process. However, preservation has seen little improvement. Existing standards use 5-10% dimethyl sulfoxide (DMSO) with serum. A new solution is needed to enable “off-the-shelf” living medicines.
cGMP CELL & GENE THERAPY
DMSO and Serum No Longer Meet Integrated Demands
Poor Performance
- Poor post-thaw cell survival as low as 15-20%
- Cell functionality impaired
Toxic
- Profound in vivo toxicity
- Severe injection site pain/neurotoxicity
- Adverse Genotoxicity
Nature, Scientific Reports volume 9, Article number: 4641 (2019)
Hindered Scalability
- Processing nightmare
- Limited scale-up production
- Batch variation
Biopreservation is the key for commercial cell manufacturing & processing, which happens 3-5 times throughout the process. However, preservation has seen little improvement. Existing standards use 5-10% dimethyl sulfoxide (DMSO) with serum. A new solution is needed to enable “off-the-shelf” living medicines.
In Vitro Fertilization
Can we say “No” to toxins for healthy babies?
DMSO
Adverse Toxicity & Genotoxicity
Poor Functional Recovery <10% (human eggs)
In Vitro Fertilization uses a high concentration of toxic vitrification media to freeze eggs or embryos that can kill a cat just in one teaspoon taken orally. This causes severe cell shrinkage and damage to the cell membrane, ultimately leading to a negative impact on the viability of the eggs, giving less than 10% with functional recovery.
The extreme changes in microRNAs and alterations in the epigenetic landscape indicate that DMSO is not inert. Its use should be reconsidered, especially for preservation of embryos and oocytes, since it may impact embryonic development.
Nature, Scientific Reports volume 9, Article number: 4641 (2019)
With X-Therma’s biopreservation breakthroughs, we make a difference.


"Look deep into nature, and then you will understand everything better. "
-Albert Einstein
As a fundamental principle of nature, reducing temperature extends time, but we had to find a way around the detrimental impact of ice crystals. Inspired by antifreeze proteins (AFPs) produced by arctic species who thrive in sub-zero environments, we developed a chemically defined antifreeze molecule that is hyper effective, non-toxic, biocompatible, and scalable.


XT-Thrive®
Chemically Defined Safe & Effective Cryopreservation
XT-Thrive® is a DMSO-free, serum-free, protein-free, and chemically defined cryopreservation media for the preservation of mammalian cells at ultra-low temperatures (-70° C to -196° C). It is a non-toxic & hyper-effective alternative to DMSO and serum products. It is plug and play for cell & tissue manufacturing processes.
YOU NOW HAVE THE TIME WITH
XT-ViVo® Preservation

NON-TOXIC
ICE-FREE




“No additional steps or equipment were needed. I used exactly the same methods as in clinical kidney transplantation.”
— Gerald Brandacher, MD, FAST; VP of Research and Scientific Director, Johns Hopkins Reconstructive Transplantation Program
MAKE TRANSPORT EASY WITH
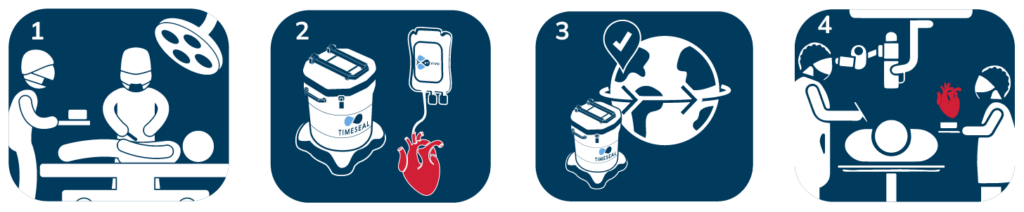
TimeSeal® Smart Sensored Organ Transporter
TimeSeal® is a smart, portable, and easy-to-use transporter to safely and consistently transport organs. With its smart sensor technology, the organ can be tracked during transport with optimum temperature stability. TimeSeal® maintains functionality and viability for organs and for a wide range of therapeutic needs. TimeSeal® doesn‘t need any external power, perfusion, oxygen, or blood to keep the organs and tissues alive.
Effective. Controlled. Simple.

“No additional steps or equipment were needed. I used exactly the same methods as in clinical kidney transplantation.”
— Gerald Brandacher, MD, FAST; VP of Research and Scientific Director, Johns Hopkins Reconstructive Transplantation Program
Simple transplant workflow